| |
Making IVF Affordable by Aniruddha Malpani
Making IVF affordable
by Aniruddha Malpani, MD
Introduction
IVF and related assisted reproductive technologies (ART) offer great hope to
infertile couples the world over. Because these techniques are so expensive,
however, they are out of the reach of the vast majority of couples, and
especially those in the developing world. This is because IVF programmes are too
technology-intensive at present - anything which is complicated is bound to be
expensive.
A high-tech approach is especially counterproductive in the developing world,
where doctors usually blindly duplicate what foreign IVF programmes do. They
imitate the Western ideal that is so tempting with its sophisticated equipment,
adopting the "never mind the cost" attitude. If this approach were successful,
then there would be little to criticize, but it can never be practical because
the infrastructure to support such sophisticated services is simply not
available in the developing world. Thus, for example, it is easy to buy an
imported CO2 incubator or a reverse-osmosis water-preparation system, but
without maintenance and after-sales services to keep them functioning properly,
the result is that these systems often become white elephants.
IVF has developed and evolved in two different directions today. One is the
high-tech approach, which includes such glamorous techniques such as
microinjection, pre-implantation genetic diagnosis, and embryo co-cultures.
These "second generation IVF procedures" are very expensive and labour-intensive.
However; they are applicable to few patients, and while worthwhile in advanced
IVF laboratories in the West, they are not relevant in the developing world,
where the basic goal of an IVF clinic is to provide service for infertile
patients. The other direction in which IVF is evolving is towards
simplification. While it is true that these "simplified IVF techniques" do not
yet offer as good a pregnancy rate as conventional IVF, they are much more
relevant in the developing world. What have these simplifications been?
Natural cycles
A major expense of the IVF cycle is the cost of the gonadotropin injections
used to induce superovulation. Superovulation using GnRH (gonadotropin-releasing
hormone) analogs and hMG (human menopausal gonadotropin) has now become the norm
for most clinics, since stimulated cycles produce more eggs and, therefore, more
embryos and a higher pregnancy rate. However, superovulation does not just carry
the risk of ovarian hyperstimulation (a potentially life-threatening condition in which the ovaries become very
enlarged because of the multiple follicles), but it also carries the risk of
multiple pregnancies and the related problem of what to do with the unwanted
eggs and embryos. Therefore, a number of clinics are now returning to the
"natural," unstimulated cycle for IVF, which is much less expensive! (1) Many
clinics are also finding that using clomiphene for gentler ovarian stimulation
gives good results for younger patients. Since most women in developing
countries get married young, the average age of infertile couples is much less
in India than it is in the West, making these protocols much more suitable for
Indian conditions.
The major problem with the original natural cycle protocol was that doctors
used to wait for the spontaneous LH surge. This meant frequent monitoring for LH
levels, and the need to be ready to do egg pickups at all hours of the day or
night. However, newer protocols using the natural cycle allow ovulation to be
induced with hCG (human chorionic gonadotropin), which in turn allows a
physician to time the egg pickup during the day. IVF is now turning full circle
- remember, the egg of the first test-tube baby was in fact recovered in a
"natural" cycle. The only disadvantage of this method is that the cancellation
rate of treatment is higher, because some eggs will ovulate before the time of
egg retrieval.
Transport IVF
A good IVF programme needs laboratory services of high standard to ensure
that the eggs, sperm, and embryos are maintained in an optimal environment in
vitro. This has been the major stumbling block for most IVF programmes. The
major limiting factor to providing IVF services has been the availability of IVF
laboratory expertise. The method of transport IVF, as described by Kingsland
(2), offers a very attractive solution to this problem. Basically, this means
that egg pickups are performed in peripheral clinics and hospitals, and the
husband transports the follicular fluid (with the eggs) to the central IVF
laboratory using a specially designed incubator which runs off the car battery.
All IVF laboratory procedures, and later the embryo transfer, are carried out in
the central laboratory.
This method allows gynecologists to take an active part in their patients'
treatment, ensuring high quality. Since all laboratory procedures are performed
in a central IVF laboratory, it also allows one IVF laboratory to obtain the
necessary experience and expertise that is so important for maintaining high
pregnancy rates.
Commercial culture medium
Making IVF culture medium in which the eggs and embryos are nourished in
vitro is a major problem. Not only is very expensive equipment needed to produce
this medium, but scrupulous quality control and testing is needed to ensure that
each batch can maintain embryo growth. With the recent commercial availability
of quality-controlled and tested culture medium - for example, Scandinavian IVF,
Menezo B2, Medicult IVF Medium, and Human Tubal Fluid - IVF programmes no longer
need to make their own culture medium, as it can now be bought "off the shelf."
This has helped to minimize the possibility of poor quality culture medium,
which used to be the major challenge responsible for reducing pregnancy rates in
IVF programmes in the past.
Vaginal incubation
Incubating the eggs and embryos in vitro requires expensive CO2 incubators,
which must maintain just the right environment for the embryos for long periods
of time. The method of intravaginal culture (IVC), however, allows one to
provide IVF services without using a CO2 incubator and is an extremely
attractive alternative. (3) Basically, in IVC the eggs and sperm are placed in
culture medium in a sterile vial, which is hermetically sealed and then placed
in the woman's vagina where it is held in place with a vaginal diaphragm. This
means that the woman acts like her own IVF incubator and keeps her embryos at
the right temperature -- 37°C . This method requires less handling of eggs and
embryos and provides a fertilization rate comparable to that of conventional
IVF, but at much less expense.
Transcervical transfer
Perhaps the ultimate simplification in IVF is the method of transcervical
oocyte-sperm transfer. As the name suggests, this simply involves transferring
the eggs (oocytes) and sperm back to the uterine cavity through the cervix after
egg pickup. (4) The rationale behind this method is that fertilization will take
place in the uterine cavity, and the resulting embryo will then implant here.
While studies of this procedure have been very preliminary, much research in
this area is currently underway.
GIFT
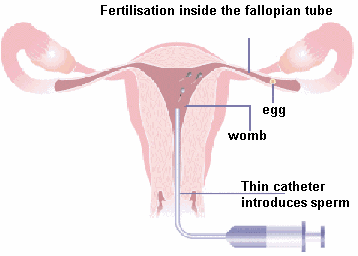 While the standard technique for women with blocked tubes has been IVF, the
method of GIFT (gamete intrafallopian transfer) developed by Asch (5) is the
method of choice for women with non-tubal infertility. In this method the eggs
and sperm (gametes) are transferred directly into the fallopian tubes (which is
where they "belong"). Pregnancy rates with GIFT are higher than IVF because the
human fallopian tubes provide a more physiological milieu for the gametes. GIFT
also requires less laboratory expertise than IVF, since in vitro gamete handling
is minimized. A major limitation with GIFT was the need to perform a laparoscopy
in order to transfer the gametes into the tubes. However, Jansen (6) has
developed special catheter sets that allow the gametes to be introduced into the
tubes under ultrasound guidance, thus making "vaginal GIFT" a non-surgical
procedure and serving to reduce its expense. While the standard technique for women with blocked tubes has been IVF, the
method of GIFT (gamete intrafallopian transfer) developed by Asch (5) is the
method of choice for women with non-tubal infertility. In this method the eggs
and sperm (gametes) are transferred directly into the fallopian tubes (which is
where they "belong"). Pregnancy rates with GIFT are higher than IVF because the
human fallopian tubes provide a more physiological milieu for the gametes. GIFT
also requires less laboratory expertise than IVF, since in vitro gamete handling
is minimized. A major limitation with GIFT was the need to perform a laparoscopy
in order to transfer the gametes into the tubes. However, Jansen (6) has
developed special catheter sets that allow the gametes to be introduced into the
tubes under ultrasound guidance, thus making "vaginal GIFT" a non-surgical
procedure and serving to reduce its expense.
Conclusion
Our personal philosophy towards assisted conception (7) is to try to keep it
as simple and cheap as possible. We are willing to accept lower pregnancy rates
per attempt, but since our patients can afford many more attempts, our
cumulative conception rate is quite good. If the cost-effectiveness of treatment
is considered (the number of 'take-home babies' per dollar spent) then our
cost-effectiveness is comparable to the best in the world. While it may be true
that patients may take longer to get pregnant, they spend much less money in the
long run. Most importantly, our approach makes IVF services available to couples
who could never have even dreamed of making a single attempt in any other centre
because of the high expenses involved.
Simplified protocols are also much more "patient-friendly." Since
conventional IVF is so expensive, going through the process is very stressful
for patients. The monitoring is very intensive and disrupting. Since so much
money is at stake, patients are very apprehensive of the outcome, and are
distressed if the cycle fails.
Moreover, since the treatment cycle is so expensive, few patients can afford
to repeat it, so most have to drop out without succeeding in getting pregnant.
On the other hand, if treatment was simple and inexpensive, patients could be
counseled to view each attempt much as an insemination cycle is viewed today -
something to be repeated as needed until the goal is reached. Aside from
providing an additional alternative for most patients, this offers a much more
realistic option. This would reduce stress and anxiety considerably, and make
treatment much more manageable for the patient.
Present-day IVF research in is focused on high-technology areas - for
example, microinjection of sperm. These techniques are very expensive,
applicable to few patients, and have poor pregnancy rates - they have reached
the point of diminishing returns. Instead, IVF research today should focus on
the further development-assisted conception, whereby better pregnancy rates can
be achieved.
References
- Foulot H et al. In vitro fertilization without ovarian stimulation: a
simplified protocol applied in 80 cycles. Fertil steril 1989;52:617.
- Kingsland C et al. Transport in vitro fertilisation --- a novel scheme for
community-based treatment. Fertile steril 1992; 58: 153.
- Ranoux C et al. A new in vitro fertilisation technique: intravaginal
culture. Fertil steril 1988; 49:654.
- Veersema S et al. Pregnancy following transcervical transfer of oocyte and
sperm. NEJM 1989; 320: 1499.
- Asch RH et al. Preliminary experiences with gamete intrafallopian
transfer. Fertil steril 1985;45:366.
- Jansen RPS, Anderson JC. Catheterisation of the Fallopian Tubes from the
Vagina. Lancet 1987;2:309.
- Malpani A, Malpani A. Simplifying assisted conception techniques to make
them universally available --- a view from India. Hum Reprod 1991;7:49.
©Dr Aniruddha Malpani, MD
Medical Director
Malpani Infertility Clinic
Bombay.

Reprinted with
permission from OBGYN.net
|



